One Reagent for All Stages of Virus Production - From Discovery Through Large-Scale Manufacturing
R&D, Preclinical, or GMP Quality; Ready-to-Use, Chemically Defined, Animal Origin Free, Free Samples Available
Transient transfection is a robust and reliable tool for the manufacture of recombinant lentivirus and adeno-associated virus (AAV). The therapeutic promise of these technologies through the modification of relevant immune cells (e.g., CAR-T Cell Therapy) or overexpression of a target gene drives the demand for enhanced virus production platforms. A key within this platform is a transfection reagent not only with high transfection efficiency but one that also yields the highest viral titers.
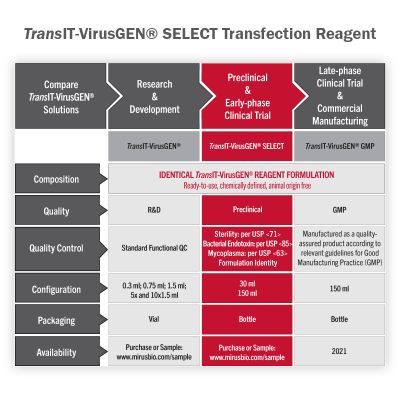
Recognizing this need, Mirus Bio has developed a novel transfection formulation, TransIT-VirusGEN®, specifically for high titer manufacture of recombinant lentivirus and AAV in 293-derived cell types. In addition, movement of recombinant virus production into the gene and cell therapy manufacturing arena increases the focus on quality parameters such as reproducibility and raw material testing.
TransIT-VirusGEN® Transfection Reagent - Ideal for Academic Research and Biopharmaceutical Discovery
TransIT-VirusGEN® is a high-performance transfection reagent for the reliable and efficient delivery of packaging and transfer vectors to adherent or suspension HEK293 cell types. With a streamlined recombinant adeno-associated virus (AAV) and lentivirus production workflow and highly efficient DNA delivery, TransIT-VirusGEN can be used with a variety of cell culture systems.
- Consistent high virus titer production
- Compatible with adherent or suspension HEK293 cells
- Can be scaled efficiently
- Streamlined virus generation workflow
- Compatible with different virus & cell culture systems
- Completely synthetic and animal origin free
TransIT-VirusGEN® SELECT Transfection Reagent - Large Scale Virus Production for Preclinical and Early Phase Clinical Trials
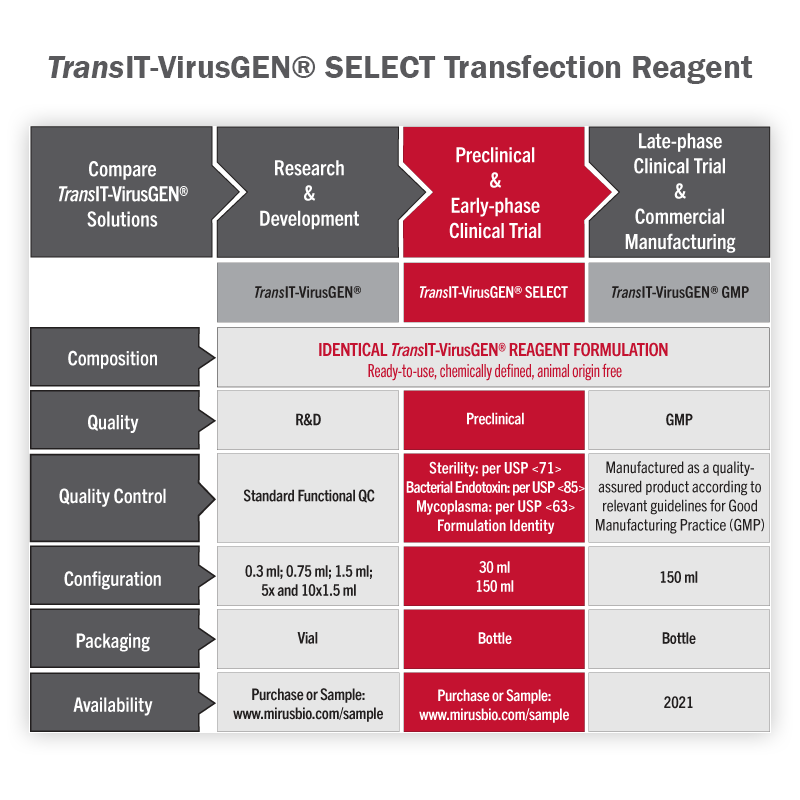
All TransIT-VirusGEN® reagents are designed to enhance delivery of packaging and transfer vector DNA to suspension and adherent HEK 293 cell types in order to increase production of recombinant lentivirus and adeno-associated virus (AAV). TransIT-VirusGEN® SELECT Transfection Reagent is identical in formulation to the research-grade TransIT-VirusGEN® and includes release testing and quality documentation to streamline the process of ancillary material qualification, ensuring seamless transitions from discovery through large-scale manufacturing. With performance that exceeds PEI and PEIpro® reagents, TransIT-VirusGEN® SELECT offers a simplified, cost-effective workflow, making it the superior choice for large-scale virus production.
- Performance - Efficient DNA delivery for large-scale production of high-titer viral vectors
- Quality - Tested for performance, identity, sterility, endotoxin and mycoplasma
- Reliability - Exceptional lot-to-lot consistency
- Cell Line and Platform Flexibility - Compatible with different virus production platforms and repeat filtration
- Animal Origin Free - Fully synthetic transfection reagent formulation
- Worry Free – No commercial license required
Coming soon:
TransIT-VirusGEN® GMP Transfection Reagent – Late-Phase Clinical Trial and Commercial Manufacturing
TransIT-VirusGEN® GMP Transfection Reagent is identical in formulation to research-grade TransIT-VirusGEN® and TransIT-VirusGEN® SELECT, but all manufacturing including the lipid and polymer components and the final formulation are carried under Good Manufacturing Practice (GMP).
TransIT-VirusGEN® Biotherapeutic Pipeline
Poster
High Titer Recombinant Lentivirus and Adeno-associated Virus Production for Therapeutic Applications
Brochure
TransIT® VirusGEN® SELECT Brochure
Video
High Titer LV and AAV Production
Order TransIT-VirusGEN®
TransIT-VirusGEN® Transfection Reagent
TransIT-VirusGEN® SELECT Transfection Reagent